7252014 An excited state means that typically the valence electron has moved from its ground state orbital ie. Neon has another excited state 196 eV below this 1870 eV above the ground.
 Ground State Vs Excited State Electron Configuration Examples
Ground State Vs Excited State Electron Configuration Examples
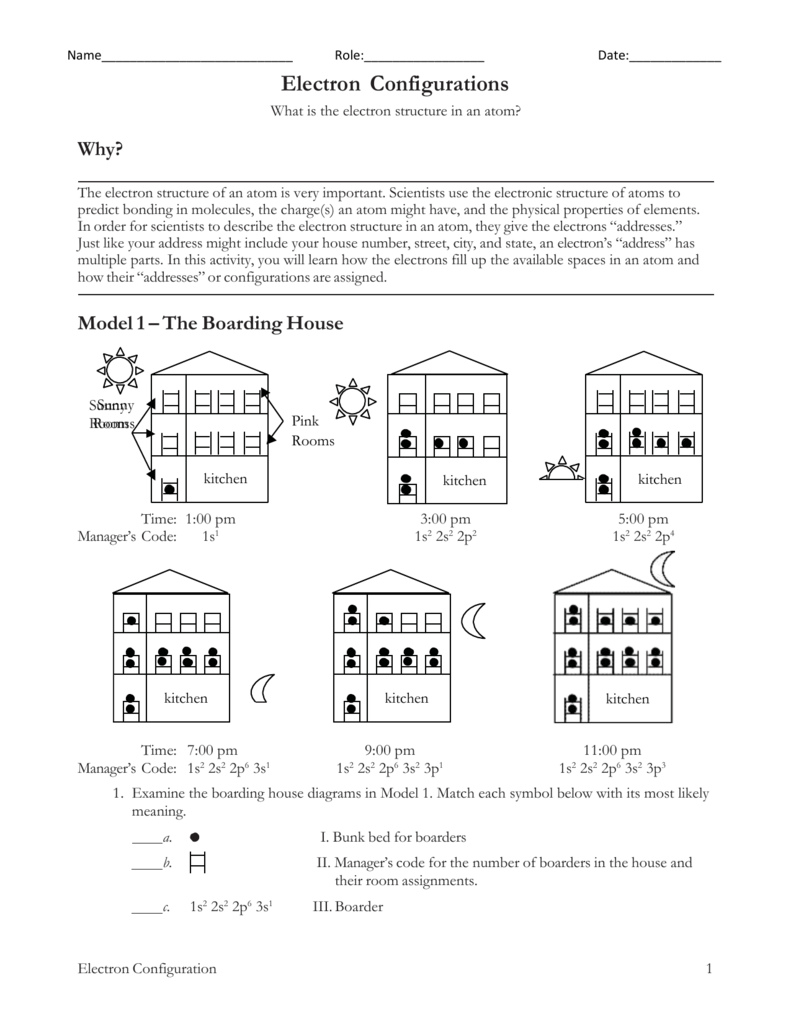
Calcium atom has an atomic number of 20 and its electronic configuration is 2 8 8 2.
Electron configuration of neon in excited state. Next we have two for 10 electrons. First give the electronic configuration for the elements by electron subshell ordering and then apply the excitation to the outer most orbital electrons of the corresponding elements. The atomic number for neon is 10 therefore.
642011 Transition of neon from ground state to excited state. Atoms can move from one configuration to another by absorbing or emitting energy. Flourine has 9 electrons but F has gained an electron and thus has 10.
Which electron configuration represents a neutral atom of nitrogen in an excited state. The ground-state electron configuration is Ne3s 2 3p 4. A step-by-step description of how to write the electron configuration for Neon Ne.
An excited state of this element has the electron configuration Kr5s 2 4d 6 5p 2 6s 1. Count the electrons and see that the total still adds up to. This will give you the term symbol for the ground state.
In order to write the Ne electron configuration we first need to know t. For example an atom in an excited state may contain two electrons in its 1s orbital one electron in its 2s orbital and one electron in each of its 2p orbitals. An excited state of this element has the electron configuration 1s 2 2s 2 2p 5 3s 1.
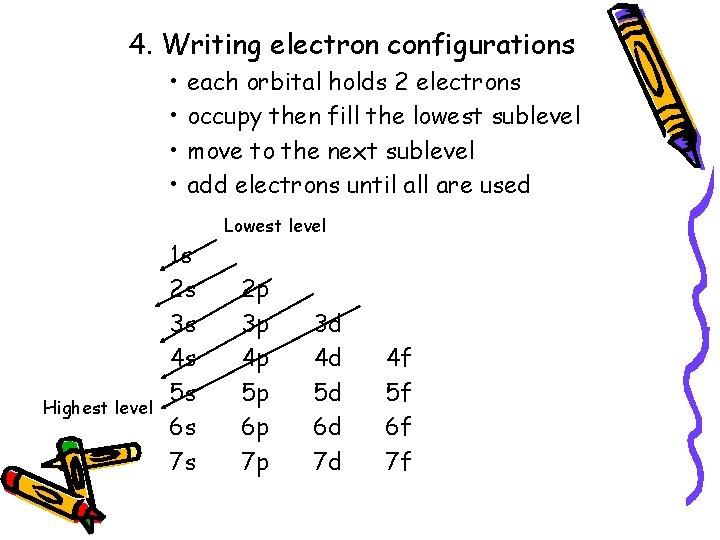
First you need to have the ground state electron configuration for Neon this is 1s 2 2p 6 or what ever it is for your atom. 1s 2 2s 1 2p 5. The electron configuration is the same as for neon and the number of nonvalence electrons is 2.
Neon has lowest excited energy at 1 s22 s22 p53 s1 state. The remaining six electrons will go in the 2p orbital. And next energy is at 1 s22 s22 p53 p1 state.
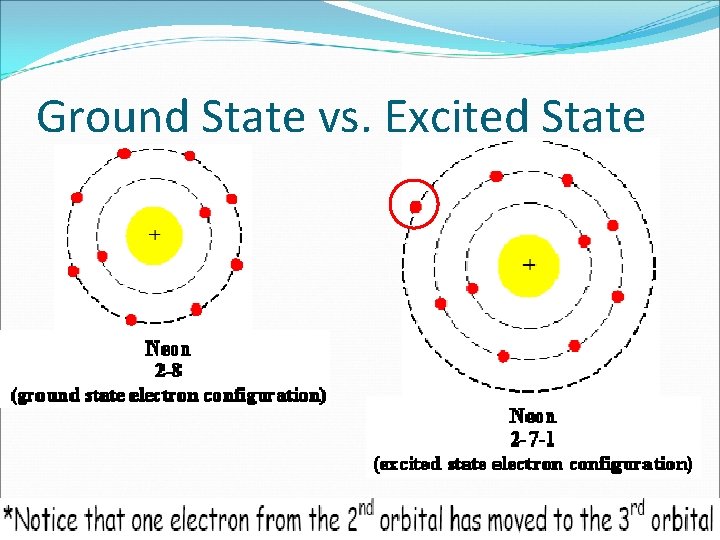
An atom is said to be in an excited state if it gains energy and move to an higher energy level. 3272013 The presence of an odd number of electrons in a lower sublevel is a tip-off that you are looking at an excited configuration. An impossible electron configuration.
1252011 An electron configuration is in an excited state when the oribital is not completely full. Lowest available energy to some other higher energy orbital. The first excited state is obtained by promoting a 3s electron to the 3p orbital to obtain the 1s 2 2s 2 2p 6 3p 1 configuration abbreviated as the 3p level.
3172017 The first excited state is obtained by promoting a 3s electron to the 3p orbital to obtain the 1s22s22p63p configuration abbreviated as the 3p level. As an example the ground state configuration of the sodium atom is 1s 2 2s 2 2p 6 3s 1 as deduced from the Aufbau principle see below. Which is the electron configuration for a neutral atom in the.
Neon is the tenth element with a total of 10 electrons. An excited state of neon. Step 1 of 5.
Excited state of neon atom Atomic number of the given element 10 Electronic configuration 1s22s2p6 1s22s22p6 is electronic configuration of Ne. 1s 2 2s 2 2p 6 3s 2 3p 5 4s 1 could be the electron configuration of. So any electron configuration in which the last electron again the valence electron is in a higher energy orbital this element is said to be in an excited state.
And the excitation energy is about 19eV. And so this is neon and this is neon Indy Ground State. So we have some for this one.
The atom whose outermost shell structure principal energy level most closely resembles that of neon atomic number 10 has the atomic number _____. Thus the number of nonvalence electrons is 2 10 total electrons 8 valence. The ground state of.
The two rules for filling the electronic. Eneon 13Potassium ion K has the same electronic structure as a neutral atom of A1s22s22p63s3 B1s22s22p63s23d1 C1s22s22p63s23p1 D1s22s22p63s23d64s24p1 E1s22s22p63s23p2 14In the excited state a possible electron configuration of aluminum 13Al is Asodium ion Bmanganese atom Ccalcium atom in the ground state Dsodium ion in an. 2 2s 1 2p 4.
The excited state has one electron promoted say p to a d orbital then 1s 2 2p 5 3d 1. Any other configuration is an excited state. Z eff Ne 10 2 8.
And the excitation energy is about 169eV. The ground-state electron configuration contains three unpaired 6p electrons. And the actual electron configuration is one es tu tu es tu and true p one not three people.
We have 121245 But electrons. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. Atoms can move from one configuration to.
Additional thermal motion of the helium and neon atoms allow such an excited helium atom to collide or interact with neon atoms and put these neon atoms into their 2066 eV excited states. The electron configuration that represent an excited state for an atom of calcium is 2 8 7 3. So that means that were dealing with born from these excited state.
The electron configuration for an O2- ion is 1s22s22p6 just like the noble gas neon. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. 842015 An excited state differs from a ground state which is when all of the atoms electrons are in the their lowest possible orbital.
The ground state of potassium. None of the previous answers. The element with the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 is.
 Excited State Of An Atom Electron Configuration
Excited State Of An Atom Electron Configuration
 Aim What Happens When Electrons Get Excited Do
Aim What Happens When Electrons Get Excited Do
 Study The Structure Of The Atom With Paper Plates M M S This Lab Investigation On Edible Electrons And Elements Atom Activities Paper Plates Periodic Table
Study The Structure Of The Atom With Paper Plates M M S This Lab Investigation On Edible Electrons And Elements Atom Activities Paper Plates Periodic Table
 Iii Electron Structure A Energy Levels Energy Levels
Iii Electron Structure A Energy Levels Energy Levels
 Electron Configuration Of Sodium Learn Lif Co Id
Electron Configuration Of Sodium Learn Lif Co Id
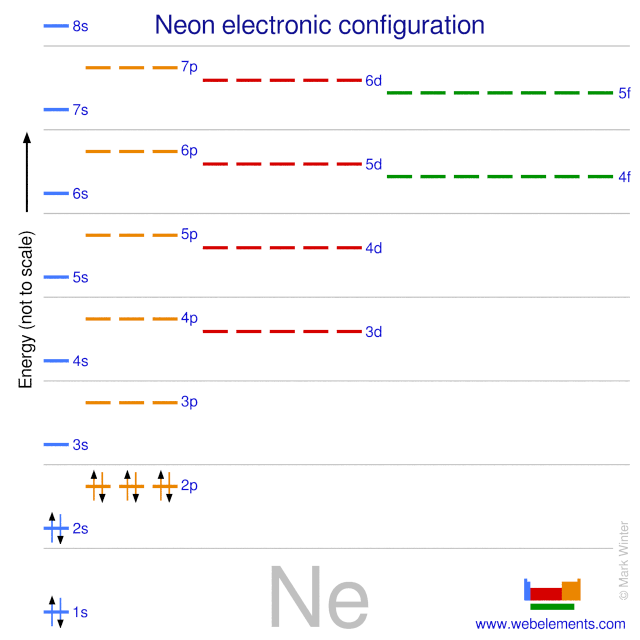 Webelements Periodic Table Neon Properties Of Free Atoms
Webelements Periodic Table Neon Properties Of Free Atoms
 Topic Atomic Concepts Aim What Is The Difference Between Ground State And Excited State Electron Configurations How Can We Identify Unknown Elements Ppt Download
Topic Atomic Concepts Aim What Is The Difference Between Ground State And Excited State Electron Configurations How Can We Identify Unknown Elements Ppt Download
 Calcium Electron Configuration Youtube
Calcium Electron Configuration Youtube
 Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
 Neon Electron Configuration Youtube
Neon Electron Configuration Youtube
 Science Project Atom Model Shape Of Pizza Extremely Easy Cheap Atomlar
Science Project Atom Model Shape Of Pizza Extremely Easy Cheap Atomlar
 Aim What Happens When Electrons Get Excited Do
Aim What Happens When Electrons Get Excited Do
 Sp2 And Sp Hybridization High School Chemistry Chemistry Boron Atom
Sp2 And Sp Hybridization High School Chemistry Chemistry Boron Atom
 Difference Between Ground State And Excited State
Difference Between Ground State And Excited State
 Aufbau Principle Png Images Pngwing
Aufbau Principle Png Images Pngwing
 Electron Configuration Worksheet Docx Electron Configurations What Is The Electron Structure In An Atom Why The Electron Structure Of An Atom Is Very Course Hero
Electron Configuration Worksheet Docx Electron Configurations What Is The Electron Structure In An Atom Why The Electron Structure Of An Atom Is Very Course Hero
Write An Electron Configuration For A Silicon Atom In An Excited State